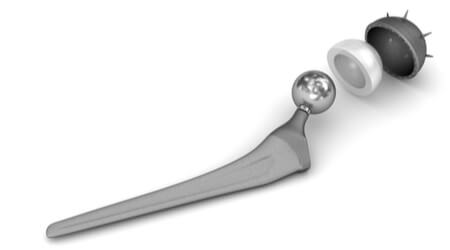
Stryker® Orthopaedics has issued a voluntary recall of its Rejuvenate Modular and ABG-II modular-neck hip stem implants, according to the Food and Drug Administration (FDA).
Corrosion can occur in the devices, and this wear and tear can release metal debris that can cause adverse tissue reactions and pain and swelling, according to Stryker.
Although the hip sockets in these models are not metal-on-metal joints, the recalled hip implants do contain a metal-on-metal joint within their workings. The recalled devices can deteriorate over time, shedding small particles of metal.
Some of the metal ions (such as cobalt and chromium) from the metal implant or from the metal particles can enter the bloodstream. This can lead to metallosis, a condition that results from a dangerous accumulation of metal debris in the soft tissues of the body.
In order to address the failure of the prosthetic hip, as well as any physical damage related to metallosis, patients who have received Stryker hip implants may require hospitalization and painful corrective surgeries. Left untreated, metallosis can potentially cause heart and kidney damage and neurological and psychological changes.
Stryker’s recall notice encouraged recipients of Rejuvenate Modular or ABG II modular-neck stems to contact their surgeons.
Which Stryker Hip Implants are the Subject of the Voluntary Recall?
Stryker Orthopaedics produces a variety of medical devices and implants. The hip implants targeted by this specific recall are the:
- Rejuvenate Modular Hip System
- ABG II modular-neck hip stem
In an October 2013 regulatory filing, Stryker said it expects to spend between $700 million and $1.13 billion to resolve litigation and other costs related to its hip implant recall. Stryker has said that it intends to reimburse patients for “reasonable and customary” medical costs, including revision surgeries.
If you are unsure whether you received one of the recalled hip implants, you should contact your surgeon or review your medical records.
Hip Implants Could Pose Serious Health Risks
Patients who received a recalled Stryker hip implant should make an appointment with their surgeon as soon as possible if they are experiencing pain or swelling in their hip region.
Fretting and corrosion are not outwardly visible because the components are implanted in the body, but there are physical side effects that could indicate that your particular hip replacement is defective.
Deterioration of soft tissue (fibrosis) in the patients’ hip as the artificial joint wears away may cause the implant to come loose, which is likely to cause mobility problems in addition to pain. Patients may experience swelling, or hear grinding or popping noises from the prosthesis when they move. Metallosis can also cause a rash.
According to the FDA, some case reports and medical studies have suggested that metallosis may cause systemic reactions (symptoms or illness elsewhere in the body), such as:
- Cardiomyopathy (disease of the heart muscle)
- Renal (kidney) function impairment
- Neurological changes, including hearing and vision problems
- Psychological changes, including depression or cognitive (thought, memory, attention, etc.) impairment
- Thyroid dysfunction, including neck discomfort, fatigue, weight gain, or feeling cold.
People react differently to metallosis. There’s no way to tell how much of a buildup of loose metal in your body may occur before it adversely affects you or how it will affect you. In order to avoid or correct problems related to the recalled Stryker hip implants, it might be necessary for you to undergo a painful revision operation.
Recalled Stryker Hip Implant? You Could Be Entitled to Compensation
If you or someone you love received a recalled Stryker Orthopaedics hip implant and suffered painful swelling or related side effects that resulted in hospitalization or revision surgery, contact Joye Law Firm today. Our South Carolina defective medical device attorneys may be able to help you get the compensation that you deserve for your recalled hip implant injury.
Call Joye Law Firm or fill out our online contact form for a free case evaluation.
Stryker® is a registered trademark of Stryker Corporation. This law firm is not associated with, sponsored by, or affiliated with the U.S. Food and Drug Administration or Stryker Corporation.